Organic Chemistry Division and Central NMR Facility
CSIR-National Chemical Laboratory (NCL)
Peptides-NMR-Honey Science Group
Publications
Peptides - NMR

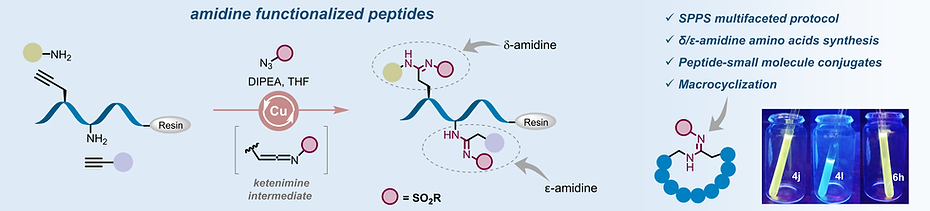
-
Ketenimine Multicomponent Strategy for Multifaceted Amidine Functionalization of Peptides on the Solid Phase
Bodake SM, Marelli UK
Angew. Chem. Int. Ed. 2025, e202509854.
https://onlinelibrary.wiley.com/doi/full/10.1002/anie.202509854
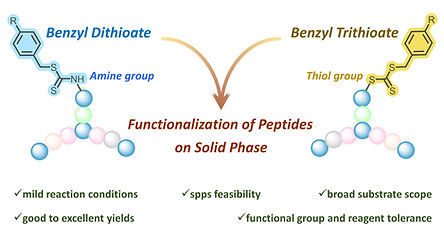

-
Peptide Functionalization with Dithioate and Trithioate Groups: A CS2-Mediated Solid-Phase Approach
Shinde DR, Bodake SM, Marelli UK
Org. Lett. 2025, 27, 24, 6271–6278. (Front Cover Article).

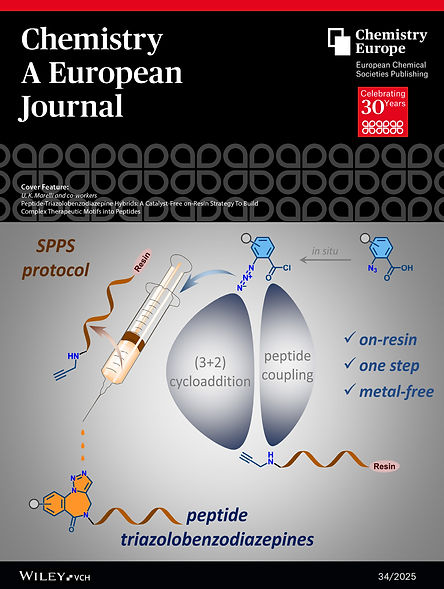
-
Peptide-Triazolobenzodiazepine Hybrids: A Catalyst-Free on-Resin Strategy to Build Complex Therapeutic Motifs Into Peptides
Kamble SSM, Bodake SM, Marelli UK
Chem. Eur. J. 2025, 31, e202500836. (Cover Feature Article).
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.202500836
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.202583403
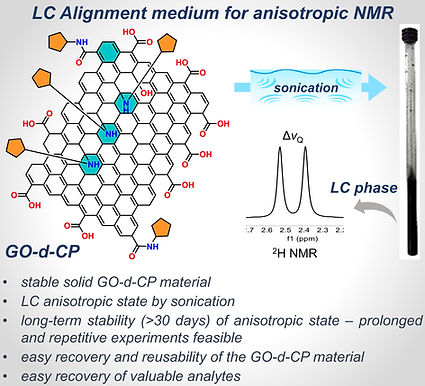
-
Graphene Oxide-Cyclopentylamine (GO-d-CP) Liquid Crystals as a Novel Alignment Medium for Anisotropic NMR with Analyte Recovery
SC Jatheendranath, A Das, SH Deshpande, N Nath, W Maier, V Streitferdt, Marelli UK
Chem. Methods 2025, , e202400039.
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cmtd.202400039?af=R
-
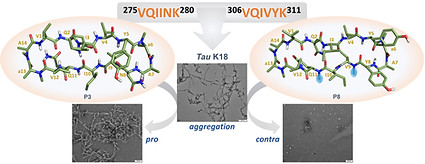
Macrocyclic peptides derived from AcPHF6* and AcPHF6 to selectively modulate the Tau aggregation
Dangi A, Qureshi T, Chinnathambi S, Marelli UK
Bioorg. Chem. 2024, 151, 107625.
https://www.sciencedirect.com/science/article/pii/S0045206824005303
-
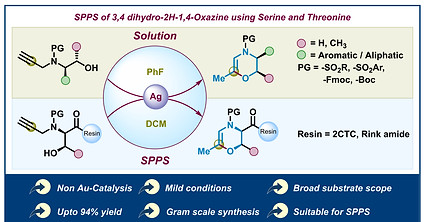
Integrating 3,4-Dihydro-2H-1,4-oxazine into Peptides as a Modification: Silver Triflate-Catalyzed Cyclization of N-Propargyl N-Sulfonyl Amino Alcohols for SPPS Applications
AR Patil, Marelli UK
Org. Lett. 2024, 26, 7584-7589.
-
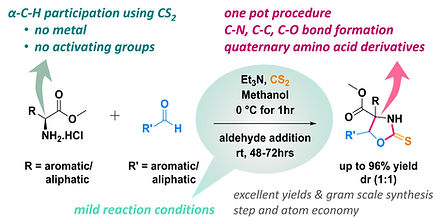
Metal-Free One-Pot Domino Synthesis of Oxazolidinethione Derivatives of Quaternary Amino Acids from α-Amino Esters and Aldehydes Using CS2
Shinde DR, Krishna GR, Marelli UK
J. Org. Chem. 2024, 89, 7109-7114.
-
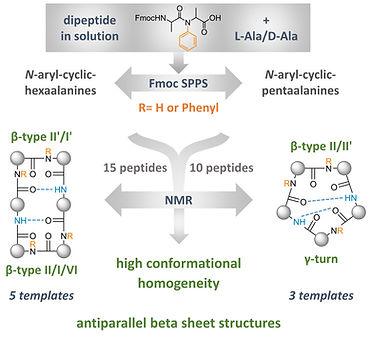
Exploration of N‐arylation of backbone amides as a novel tool for conformational modification in peptides
Dangi A, Marelli UK
Chem. Eur. J. 2023, 29, e202300753. (Cover Feature Article)
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.202301803
https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/chem.202300753
-
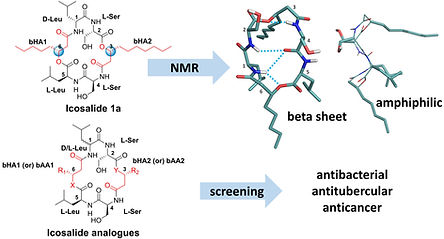
Total synthesis, structure elucidation and expanded bioactivity of icosalide A: effect of lipophilicity and ester to amide substitution on its bioactivity
Dangi A, Pande B, Agrawal S, Sarkar D, Vamkudoth KR, Marelli UK
Org. Biomol. Chem. 2023, 21, 5725-5731.
https://pubs.rsc.org/en/content/articlelanding/2023/OB/D3OB00809F

-
Residue-based propensity of aggregation in the Tau amyloidogenic hexapeptides AcPHF6* and AcPHF6
Dangi A, Balmik AA, Ghorpade AK, Gorantla NV, Sonawane SK, Chinnathambi S, Marelli UK
RSC Adv., 2020, 10, 27331-27335.RSC Adv., 2020, 10, 27331-27335.
https://pubs.rsc.org/en/content/articlelanding/2020/ra/d0ra03809a
-
Possible Fine-Tuning of Methane Activation toward C2 Oxygenates by 3d-Transition Metal-Ions Doped Nano-Ceria-Zirconia
Kanungo SS, Mishra AK, Mhamane NB, Marelli UK, Kumar D, Gopinath CS.
Inorg Chem. 2022, 61(48), 19577-19587.
https://pubs.acs.org/doi/full/10.1021/acs.inorgchem.2c03493
https://doi.org/10.1021/acs.inorgchem.2c03493
-
HDAC6 ZnF UBP as the Modifier of Tau Structure and Function
Balmik AA, Chidambaram H, Dangi A, Marelli UK, Chinnathambi S.
Biochemistry, 2020, 59(48), 4546-4562.
https://pubs.acs.org/doi/10.1021/acs.biochem.0c00585
https://doi.org/10.1021/acs.biochem.0c00585
-
Melatonin interacts with repeat domain of Tau to mediate disaggregation of paired helical filaments
Balmik AA, Das R, Dangi A, Gorantla NV, Marelli UK, Chinnathambi S.
Biochim Biophys Acta Gen Subj., 2020, 1864(3):129467.
https://www.sciencedirect.com/science/article/abs/pii/S0304416519302533
https://doi.org/10.1016/j.bbagen.2019.129467
-
EGCG impedes human Tau aggregation and interacts with Tau
Sonawane SK, Chidambaram H, Boral D, Gorantla NV, Balmik AA, Dangi A, Ramasamy S, Marelli UK, Chinnathambi S.
Sci Rep. 2020, 10(1):12579.
https://www.nature.com/articles/s41598-020-69429-6
https://doi.org/10.1038/s41598-020-69429-6
-
Molecular Networking and Whole-Genome Analysis Aid Discovery of an Angucycline That Inactivates mTORC1/C2 and Induces Programmed Cell Death
Dan VM, J S V, C J S, Sanawar R, Lekshmi A, Kumar RA, Santhosh Kumar TR, Marelli UK, Dastager SG, Pillai MR
ACS Chem Biol., 2020, 15(3), 780-788.
https://pubs.acs.org/doi/abs/10.1021/acschembio.0c00026
https://doi.org/10.1021/acschembio.0c00026
-
Novel cilengitide-based cyclic RGD peptides as αvβ3 integrin inhibitors
Meena CL, Singh D, Weinmüller M, Reichart F, Dangi A, Marelli UK, Zahler S, Sanjayan GJ
Bioorg Med Chem Lett., 2020, 30(8), 127039.
https://www.sciencedirect.com/science/article/pii/S0960894X20301116
https://doi.org/10.1016/j.bmcl.2020.127039
-
Heterocyclic Carbene Catalysis Exploiting Oxidative Imine Umpolung for the Generation of Imidoyl Azoliums
Das TK, Madica K, Krishnan J, Marelli UK, Biju AT
J Org Chem., 2020, 85(7), 5114-5121.
https://pubs.acs.org/doi/abs/10.1021/acs.joc.0c00360
https://doi.org/10.1021/acs.joc.0c00360
-
N-Heterocyclic Carbene-Catalyzed Umpolung of Imines for the Enantioselective Synthesis of Dihydroquinoxalines
Das TK, Ghosh A, Balanna K, Behera P, Gonnade RG, Marelli UK, Das AK, Biju AT
ACS Catal. 2019, 9, 5, 4065-4071.
https://pubs.acs.org/doi/abs/10.1021/acscatal.9b00737
https://doi.org/10.1021/acscatal.9b00737
-
Selective Targeting of Integrin αvβ8 by a Highly Active Cyclic Peptide
Reichart F, Maltsev OV, Kapp TG, Räder AFB, Weinmüller M, Marelli UK, Notni J, Wurzer A, Beck R, Wester HJ, Steiger K, Di Maro S, Di Leva FS, Marinelli L, Nieberler M, Reuning U, Schwaiger M, Kessler H.
J. Med. Chem. 2019, 62, 2024-2037.
https://pubs.acs.org/doi/10.1021/acs.jmedchem.8b01588
https://doi.org/10.1021/acs.jmedchem.8b01588
-
Systematic synthesis of a 6-component organic-salt alloy of naftopidil, and pentanary, quaternary and ternary multicomponent crystals
Dandela R, Tothadi S, Marelli UK, Nangia A.
IUCrJ. 2018, 5, 816-822.
http://journals.iucr.org/m/issues/2018/06/00/ed5016/index.html
https://doi.org/10.1107/S2052252518014057
-
N-Heterocyclic Carbene-Catalyzed Michael–Michael–Lactonization Cascade for the Enantioselective Synthesis of Tricyclic δ-Lactones
Mukherjee S, Ghosh A, Marelli UK, Biju AT.
Org Lett. 2018, 18, 2952-2955.
https://pubs.acs.org/doi/10.1021/acs.orglett.8b00998
https://doi.org/10.1021/acs.orglett.8b00998
-
From a Helix to a Small Cycle: Metadynamics-Inspired αvβ6 Integrin Selective Ligands
Di Leva FS, Tomassi S, Di Maro S, Reichart F, Notni J, Dangi A, Marelli UK, Brancaccio D, Merlino F, Wester HJ, Novellino E, Kessler H, Marinelli L.
Angew. Chem. Int. Ed. 2018, 57, 14645-14649.
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201803250
https://doi.org/10.1002/anie.201803250
-
N-Methylation of isoDGR Peptides: Discovery of a Selective α5β1-Integrin Ligand as a Potent Tumor Imaging Agent
Kapp TG, Di Leva FS, Notni J, Räder AFB, Fottner M, Reichart F, Reich D, Wurzer A, Steiger K, Novellino E, Marelli UK, Wester HJ, Marinelli L, Kessler H.
J. Med. Chem. 2018, 61, 2490-2499.
https://pubs.acs.org/doi/10.1021/acs.jmedchem.7b01752
https://doi.org/10.1021/acs.jmedchem.7b01752
-
Overcoming the Lack of Oral Availability of Cyclic Hexapeptides: Design of a Selective and Orally Available Ligand for the Integrin αvβ3
Weinmüller M, Rechenmacher F, Marelli UK, Reichart F, Kapp TG, Räder AFB, Di Leva FS, Marinelli L, Novellino E, Muñoz-Félix JM, Hodivala-Dilke K, Schumacher A, Fanous J, Gilon C, Hoffman A, Kessler H
Angew. Chem. Int. Ed. 2017, 56, 16405-16409.
http://onlinelibrary.wiley.com/doi/10.1002/anie.201709709/abstract
https://doi.org/10.1002/anie.201709709
-
Structural Insights into Selective Ligand–Receptor Interactions Leading to Receptor Inactivation Utilizing Selective Melanocortin 3 Receptor Antagonists
Cai M, Marelli UK, Mertz B, Beck JG, Opperer F, Rechenmacher F, Kessler H, Hruby VJ
Biochemistry 2017, Articles ASAP.
http://pubs.acs.org/doi/full/10.1021/acs.biochem.7b00407
https://doi.org/10.1021/acs.biochem.7b00407
-
Evaluation of uttroside B, a saponin from Solanum nigrum Linn, as a promising chemotherapeutic agent against hepatocellular carcinoma
Nath LR, Gorantla JN, Thulasidasan AK, Vijayakurup V, Shah S, Anwer S, Joseph SM, Antony J, Veena KS, Sundaram S, Marelli UK, Lankalapalli RS, Anto RJ
Sci Rep. 2016, 6:36318.
http://www.nature.com/articles/srep36318
https://doi.org/10.1038/srep36318
-
Total Synthesis of the Marine Natural Product Solomonamide B Necessitates Stereochemical Revision
K. Kashinath, Gorakhnath R. Jachak, Paresh R. Athawale, Udaya Kiran Marelli, Rajesh G. Gonnade, and D. Srinivasa Reddy
Org. Lett. 2016, 18, 3178-3181.
http://pubs.acs.org/doi/abs/10.1021/acs.orglett.6b01395
https://doi.org/10.1021/acs.orglett.6b01395
Honey Profiling
Loading...

Postdoctoral Research
-
Stable Peptides Instead of Stapled Peptides: Highly Potent αvβ6‐Selective Integrin Ligands
Oleg V Maltsev, Udaya Kiran Marelli, Tobias G Kapp, Francesco Saverio Di Leva, Salvatore Di Maro, Markus Nieberler, Ute Reuning, Markus Schwaiger, Ettore Novellino, Luciana Marinelli, Horst Kessler
Angew. Chem. Int. Ed. 2016, 55, 1535-1539.
http://onlinelibrary.wiley.com/doi/10.1002/anie.201508709/full
https://doi.org/10.1002/anie.201508709
-
Cis-peptide bonds: A key for intestinal permeability of peptides?
Udaya Kiran Marelli, Oded Ovadia, Andreas Oliver Frank, Jayanta Chatterjee, Chaim Gilon, Amnon Hoffman, Horst Kessler
Chem. Eur. J. 2015, 21, 15148-15152.
http://onlinelibrary.wiley.com/doi/10.1002/chem.201501600/full
https://doi.org/10.1002/chem.201501600
-
Systematic Backbone Conformational Constraints on a Cyclic Melanotropin Ligand Leads to Highly Selective Ligands for Multiple Melanocortin Receptors
Minying Cai, Udaya Kiran Marelli, Jennifer Bao, Johannes G. Beck, Florian Opperer, Florian Rechenmacher, Kaitlyn R. McLeod, Morgan R. Zingsheim, Lucas Doedens, Horst Kessler, Victor J. Hruby
J. Med. Chem. 2015, 58, 6359-6367.
http://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.5b00102
https://doi.org/10.1021/acs.jmedchem.5b00102
-
Enantiomeric Cyclic Peptides with Different Caco-2 Permeability Suggest Carrier-Mediated Transport
Udaya Kiran Marelli, Jacqueline Bezençon, Eduard Puig, Beat Ernst, Horst Kessler
Chem. Eur. J. 2015, 21, 8023-8027.
http://onlinelibrary.wiley.com/doi/10.1002/chem.201501270/full
https://doi.org/10.1002/chem.201501270
-
Receptor-Bound Conformation of Cilengitide Better Represented by Its Solution-State Structure than the Solid-State Structure
Udaya Kiran Marelli, Andreas O. Frank, Bernhard Wahl, Valeria La Pietra, Ettore Novellino, Luciana Marinelli, Eberhardt Herdtweck, Michael Groll, Horst Kessler
Chem. Eur. J. 2014, 20, 14201-14206.
http://onlinelibrary.wiley.com/doi/10.1002/chem.201403839/abstract
https://doi.org/10.1002/chem.201403839
-
Tumor targeting via integrin ligands
Udaya Kiran Marelli, Florian Rechenmacher, Tariq Rashad Ali Sobahi, Carlos Mas-Moruno and Horst Kessler
Front. Oncol. 2013, 3:222.
http://journal.frontiersin.org/Journal/10.3389/fonc.2013.00222/full
https://doi.org/10.3389/fonc.2013.00222
-
Biselectivity of isoDGR Peptides for Fibronectin Binding Integrin Subtypes α5β1 and αvβ6: Conformational Control through Flanking Amino Acids
Alexander Bochen, Udaya Kiran Marelli, Elke Otto, Diego Pallarola, Carles Mas-Moruno, Francesco Saverio Di Leva, Heike Boehm, Joachim P. Spatz, Ettore Novellino, Horst Kessler, and Luciana Marinelli
J. Med. Chem. 2013, 56, 1509-1519.
https://pubs.acs.org/doi/10.1021/jm301221x
https://doi.org/10.1021/jm301221x
-
Intestinal Permeability of Cyclic Peptides: Common Key Backbone Motifs Identified
Johannes G. Beck, Jayanta Chatterjee, Burkhardt Laufer, Marelli Udaya Kiran, Andreas O. Frank, Stefanie Neubauer, Oded Ovadia, Sarit Greenberg, Chaim Gilon, Amnon Hoffman, and Horst Kessler.
J. Am. Chem. Soc. 2012, 134, 12125-12133.
Doctoral Research
-
Backbone and sidechain methyl Ile (δ1), Leu and Val chemical shift assignments of RDE-4 (1-243), an RNA interference initiation protein in C. elegans. (Chemical shift assignments-BMRB databank accession No. 17703)
C. Sai Chaitanya, Sonu Kumar, M. Udaya Kiran*, Mandar V. Deshmukh*
Biomol. NMR Assign. 2012, 6, 143-146.
http://link.springer.com/article/10.1007%2Fs12104-011-9343-0
https://doi.org/10.1007/s12104-011-9343-0
-
Novel helical foldamers: Organized heterogeneous backbone folding in 1:1 α/Nucleoside-derived-β-amino acid sequences.
Srivari Chandrasekhar, Nayani Kiranmai, Marelli Udaya Kiran, Ambure Sharada Devi, Gangireddy Pavan Kumar Reddy, Mohammed Idris and Bharatam Jagadeesh
Chem. Commun. 2010, 46, 6962-6964.
http://pubs.rsc.org/en/Content/ArticleLanding/2010/CC/c0cc01724h
https://doi.org/10.1039/C0CC01724H
-
RDC Enhanced NMR Spectroscopy in Organic Solvent Media: The Importance for the Experimental Determination of Periodic Hydrogen Bonded Secondary Structures.
Marelli Udaya Kiran, Ambadi Sudhakar, Jochen Klages, Grit Kummerlöwe, Burkhard Luy and Bharatam Jagadeesh.
J. Am. Chem. Soc. 2009, 131, 15590-15591.
http://pubs.acs.org/doi/abs/10.1021/ja906796v
https://doi.org/10.1021/ja906796v
-
Covalently Cross-linked Gelatin Allows Chiral Distinction at Elevated Temperatures and in DMSO.
Grit Kummerlöwe, Marelli Udaya Kiran and Burkhard Luy. (equal contribution authorship)
Chem. Eur. J. 2009, 15, 12192-12195.
http://onlinelibrary.wiley.com/doi/10.1002/chem.200902193/abstract
https://doi.org/10.1002/chem.200902193
-
Backbone Regulation Mimicry by β-Peptidic Foldamers: Formation of a 10-Helix in a Mixed 6-Strand/14-Helix Conformational Pool.
Bharatam Jagadeesh, Marelli Udaya Kiran, Ambadi Sudhakar and Srivari Chandrasekhar.
Chem. Eur. J. 2009, 15, 12592-12595.
http://onlinelibrary.wiley.com/doi/10.1002/chem.200902332/full
https://doi.org/10.1002/chem.200902332
-
Stabilization of β-hairpin structures via inter-strand π-π and hydrogen bond interactions in α-, β-, γ-hybrid peptides.
Tushar K. Chakraborty, K. Srinivasa Rao, M. Udaya Kiran, B. Jagadeesh.
Tetrahedron Letters 2009, 50, 4350-4353.
http://www.sciencedirect.com/science/article/pii/S0040403909010259
https://doi.org/10.1016/j.tetlet.2009.05.024
-
β-Sugar aminoxy peptides as rigid secondary structural scaffolds.
Srivari Chandrasekhar, Chennamaneni Lohitha Rao, Marepally Srinivasa Reddy, Ganti Dattatreya Sharma, Marelli Udaya Kiran, Police Naresh, Gunturu Krishna Chaitanya, Kotamarthi Bhanuprakash and Bharatam Jagadeesh.
J.Org.Chem. 2008, 73, 9443-9446.
http://pubs.acs.org/doi/abs/10.1021/jo801810z
https://doi.org/10.1021/jo801810z
-
β-Strand mimetics: Formation of bend-strands in oligomers of enantiomeric β-amino acids.
Srivari Chandrasekhar, Ambadi Sudhakar, Marelli Udaya Kiran, Bathini Nagendra Babu and Bharatam Jagadeesh.
Tetrahedron Letters 2008, 49, 7368-7371.
http://www.sciencedirect.com/science/article/pii/S0040403908019059
https://doi.org/10.1016/j.tetlet.2008.10.031
-
Nucleoside derived amino acids (NDA) in foldamer chemistry: Synthesis and conformational studies of homooligomers of modified AZT.
S. Chandrasekhar, G. Pavan Kumar Reddy, M. Udaya Kiran, Ch. Nagesh, B. Jagadeesh.
Tetrahedron Letters 2008, 49, 2969-2973.
http://www.sciencedirect.com/science/article/pii/S0040403908004267
https://doi.org/10.1016/j.tetlet.2008.03.001
-
Nucleation of the β-hairpin structure in a linear hybrid peptide containing α-, β- and γ-amino acids.
Tushar K. Chakraborty, K. Srinivasa Rao, M. Udaya Kiran, B. Jagadeesh.
Tetrahedron Letters 2008, 49, 2228-2231.
http://www.sciencedirect.com/science/article/pii/S0040403908002670
https://doi.org/10.1016/j.tetlet.2008.02.048
-
Synthesis and Conformational Studies of a Hybrid Cyclic Peptide Based on cis-β-Furanoid Sugar Amino Acid (FSAA) and Ornithine.
Srivari Chandrasekhar, Birudaraju Saritha, Police Naresh, Marelli Udayakiran, Chada Raji Reddy, and Bharatam Jagadeesh.
Helvetica Chimica Acta 2008, 91, 1267-1276.
http://onlinelibrary.wiley.com/doi/10.1002/hlca.200890138/abstract
https://doi.org/10.1002/hlca.200890138
-
An efficient copper-aluminum hydrotalcite catalyst for asymmetric hydrosilylation of ketones at room temperature.
Kantam ML, Laha S, Yadav J, Likhar PR, Sreedhar B, Jha S, Bhargava S, Udayakiran M, Jagadeesh B.
Org Lett. 2008, 10, 2979-2982.
http://pubs.acs.org/doi/abs/10.1021/ol800616p
https://doi.org/10.1021/ol800616p
-
The Baylis-Hillman reaction: A strategic tool for the synthesis of higher-carbon sugars.
Palakodety Radha krishna, P. V. Narasimha Reddy, A. Sreeshailam, M. Uday Kiran, B. Jagadeesh.
Tetrahedron Letters 2007, 48, 6466-6470.
http://www.sciencedirect.com/science/article/pii/S0040403907013822
https://doi.org/10.1016/j.tetlet.2007.07.067
-
Synthesis and conformational studies of 3,4-di-O-acylated furanoid sugar amino acid containing analogs of the receptor binding inhibitor of vasoactive intestinal peptide.
T. K. Chakraborty, S. Uday Kumar, B. Krishna Mohan, G. Dattatreya Sarma, M. Udaya kiran, B. Jagadeesh.
Tetrahedron Letters 2007, 48, 6945-6950.
http://www.sciencedirect.com/science/article/pii/S0040403907014888
https://doi.org/10.1016/j.tetlet.2007.07.158
-
Oligomers of cis-β-norbornene amino acid: Formation of β-strand mimetics.
Srivari Chandrasekhar, Bathini Nagendra Babu, Anabathula Prabhakar, Ambati Sudhakar, Marepally Srinivasa Reddy, Marelli Udaya Kiran, Bharatam Jagadeesh.
Chem. Commun. 2006, 1548-1550.
http://pubs.rsc.org/en/Content/ArticleLanding/2006/CC/b518420g
https://doi.org/10.1039/B518420G
-
Oppolzer Sultam directed aldol as a key step for the stereoselective syntheses of antitumor antibiotic Belactosin C and Its synthetic congeners.
Gullapalli Kumaraswamy, Mogilisetti Padmaja, Bekkam Markondaiah, Nivedita Jena, Balasubramanian Sridhar, and Marelli Udaya Kiran.
J. Org. Chem. 2006, 71, 337-340.

